Saxendaphen Injection
 Saxendaphen Injection. Liraglutide 6mg/mL
Saxendaphen Injection. Liraglutide 6mg/mL
Saxendaphen Injection is
Saxendaphen Injection is as only GLP-1 analogue among the obesity treatment and it is injection that is approved by
13 countries including MFDS, FDA, and EMA.
Saxendaphen Injection is an adjuvant for weight management of adult patients with the following BMI.
≥ 27kg/㎡ (Overweight) & One or more weight-related associated diseases.
* Weight-related associated diseases: Dysglycemia (Pre-diabetes or Diabetes Type 2), Hypertension, Dyslipidemia.
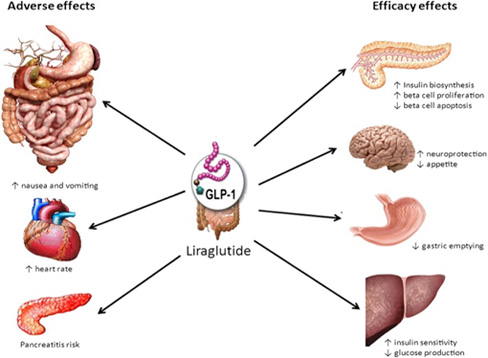
GLP-1 is as hormone secreted by the brain and intestinal L-cell
when foods are ingested, and acts on many organs.
-
- It acts on the hypothalamus of the brain and helps to anorexigenic by increase the satiety.
- In the pancreas, it encourages glucose-dependent insulin secretion by help the b-cells.
- It keeps the satiety for a long time with the reduction of gastric emptying by delay the gastrointestinal motility.
- It induces the reduction of hepatic glucose production and plasma glucagon concentration.
-
- Saxenda® is 97% similar to GLP-1, a physiological appetite regulator secreted in response to food ingestion.
- GLP-1 is secreted from the brain and intestines and acts on the hypothalamus responsible for appetite control.
- Reduces the energy intake by increasing satiety and by reducing hungry.
- With this action mechanism, patients who use Saxenda® can feel satisfied even if food intake is reduced, and can lose weight.
Weight change in 56 weeks of clinical test for 3,731 patients
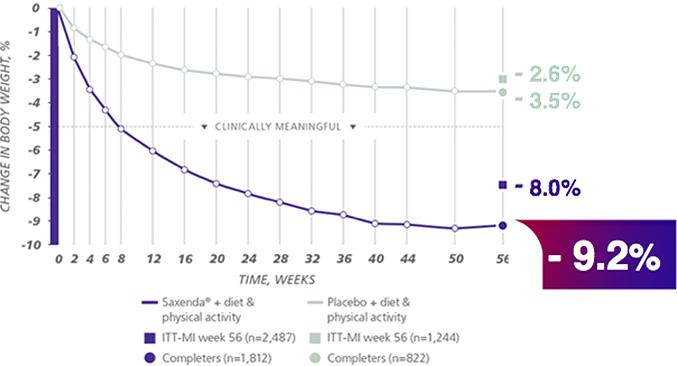

Patients treated with Saxenda® had a waist measurement reduction of 8.2 cm.
“Comparison of 5 6weeks clinical weight changes in Saxenda® and placebo users group”
In a 56-week study of 3,731 patients without diabetes and a BMI ≥30, or ≥27
with at least 1 comorbidity.
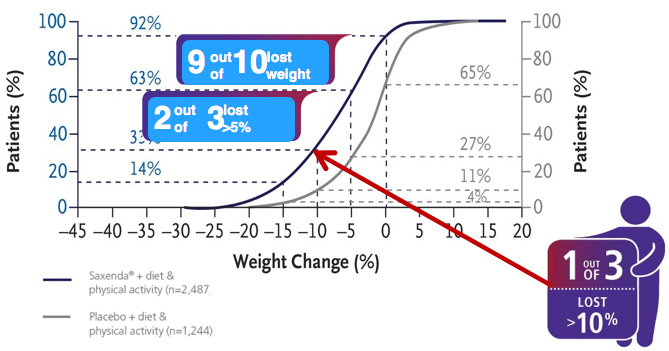
the 2 out of 3 people 5 % or more,
and the 1 out of 3 people 10% or more.
그 이상의 효과
 normal
normal
glycaemic status
glycaemic status
- In a 56-week study of 3,731 patients without diabetes and a BMI ≥30, or ≥27
with at least 1 comorbidity :
In a subset of patients with obesity and pre-diabetes(elevated glucose)
randomised to Saxenda (n=1528)
Patients in the pre-diabetic group were divided into two groups, one group treated with Saxenda® and the other group observed natural progress. In the group those who observed by natural progress, the diabetes occurred in 11% of the patients, whereas, in the Saxenda® injection group, diabetes occurred just only in 3%, and the result shows that there is 3 times reduction effect in the Saxenda® injection group. The rate that blood-sugar of pre-diabetic patients returned to normal blood-sugar reached approximately 69%.
Regardless BMI, Obesity, pre-diabetes patients experience weight loss, and improved blood sugar regulation. In addition to the improvement of diabetes rates, there are reduction effect of risk group for stroke, myocardial infarction, and cardiovascular death.
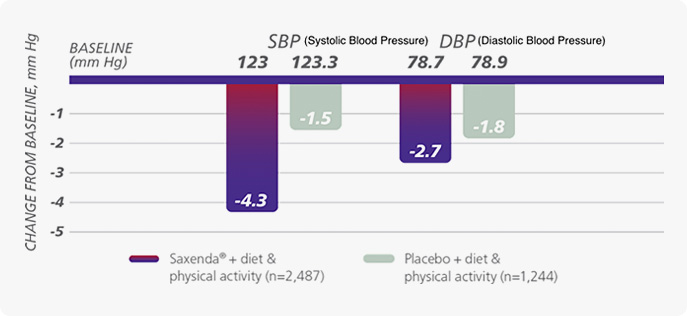
“ The Saxenda® proved cardiovascular stability because it showed a reduction effect of blood pressure of about 4 mm Hg in systolic blood pressure. ”

Saxenda provided significant reductions in blood pressure vs placebo.
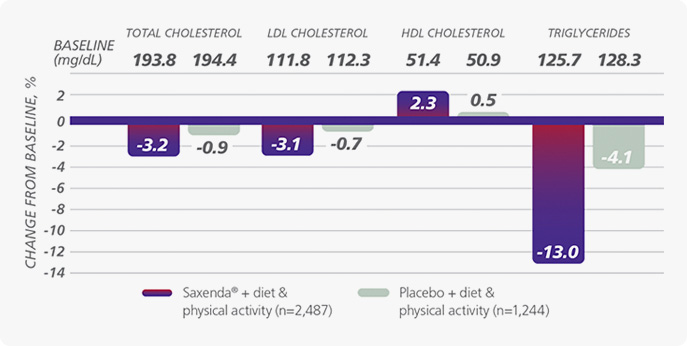
“ By improving lipid levels, lowering bad cholesterol LDL levels and elevating good cholesterol HDL levels. ”
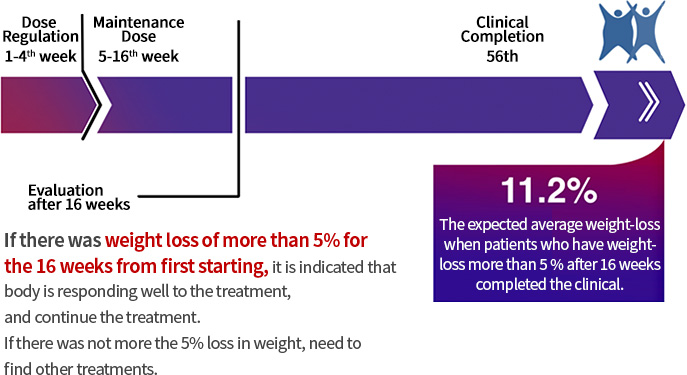
Evaluate the effectiveness of Saxendarphen® after 23 weeks from using of maintenance dose.
Administration Plan

- Gradual dose increment can minimize the nausea feeling that can occur when starting the Saxenda® first.
- As the dose increases, the body adapts to the drug and reduces nausea.
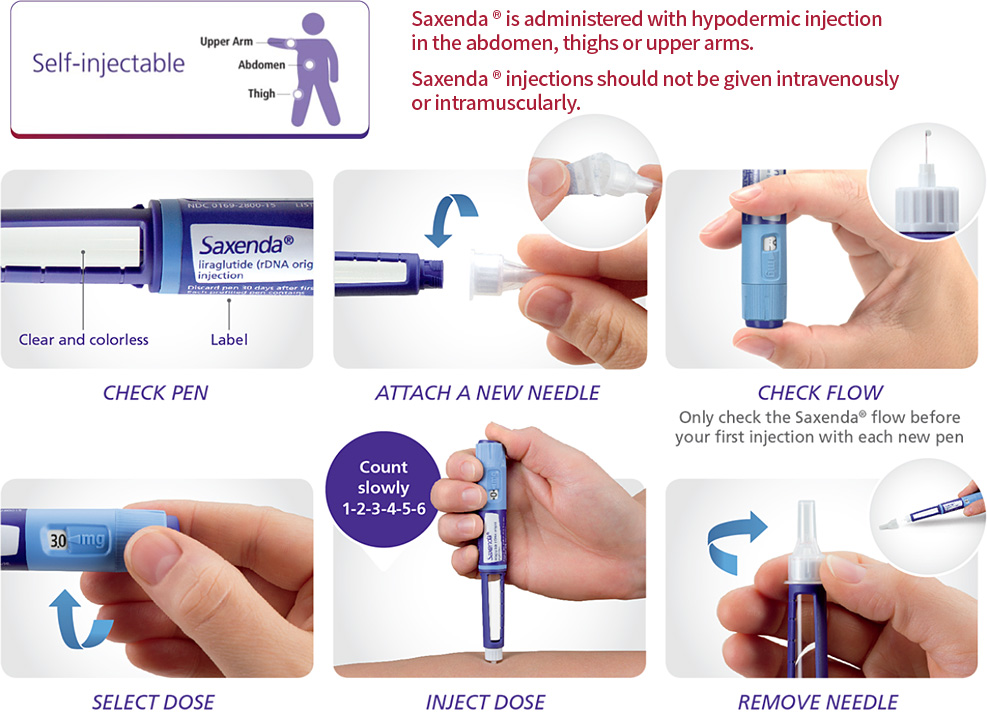
Injection Method
- Product Name Saxendaphen Injection 6mg/mL
- Contect Liraglutide 6mg/mL
- Classification · Code Special Medicines/ Metabolic medicines not classified separately
- Properties A pen-shaped free-field injection with the transparent liquid of colorless or almost colorless, (minimum dose regulation unit: 0.6 mg).
-
Effect · Efficay
This medicine is administered as an adjunct to calorie reduction diet and physical activity enhancement for the weight control of adult patients as follows:
- Obese patients with initial BMI(Body Mass Index) more than 30kg/m2, or
- Overweight patient those who have initial BMI(Body Mass Index) 27 kg / m2 or more and less than 30 kg / m2 with one or more weight-related associated disease [e.g, Dysglycemia (pre-diabetes or Diabetes Type 2), hypertension or dyslipidemia].
Treatment of this medicine should be discontinued if there is not initial weight loss of more than 5 % for 12 weeks of administration at dose of 3.0 mg/day. -
Usage · Dose
The starting dose is 0.6 mg, once a daily. For the improvement of gastrointestinal dose tolerance, the dose should be increased up to 3.0 mg once a daily by an increase of 0.6 mg at intervals of at least one week.
If a gradual increment to the next dose level does not have a dose tolerance for two consecutive weeks, discontinuation of treatment is considered. A dose more than 3.0 mg per day is not recommended. - Packing Unit 3ml / 1pen. 5pen / box

 Korean
Korean PRODUCTS
PRODUCTS 